Is Cat Litter Actually A Good Dehumidifier? We Put It To The Test
We may receive a commission on purchases made from links.
When I recently read an article online implying that a bowl of cat litter could dehumidify a bathroom and prevent mold growth, I admit that I rolled my eyes. The piece seemed to claim that one or two bags of litter could effectively dehumidify an attic or basement and that they would only need to be replaced monthly. It's one thing to say a thing "freshens your toilet" or "reduces odors," but such optimistic speculations about humidity have actual implications for people's health and the well-being of their homes. The attic claim in particular is silly; attics are open to the outdoors, and it's going to take more than two bags of cat litter to dehumidify the outdoors. So, of course, I needed to check this out.
It's true that sodium bentonite clay — the stuff that clay cat litter is usually made from — can adsorb 10-20 times its weight in water, depending on who you ask. These figures are sometimes cited to explain why litter is a good desiccant. But the problem is that litter absorbs far less moisture from the air than it does when in direct contact with liquid. There are differences in adsorption capacity between silica gel and bentonite clay, mostly related to relative humidity, but as a whole, the performance of the two desiccants is very similar when dealing with water vapor.
Preparing to test cat litter... without cats
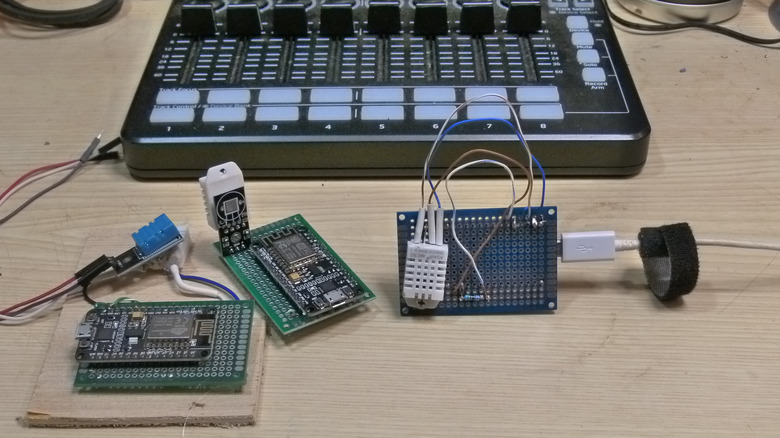
I decided to give the experiment its best shot, testing both silica gel litter (Ultra Micro Crystals cat litter) and clay (Publix brand unscented cat litter). Silica gel is perhaps the best-known desiccant (it's what's in those little packets you're not supposed to eat), and it performs well at removing moisture from the air in closed containers. Bentonite clay actually has the potential to perform even better than silica gel, but it's fussier and can contribute all of the water it captures back to the air if the ambient temperature rises too high.
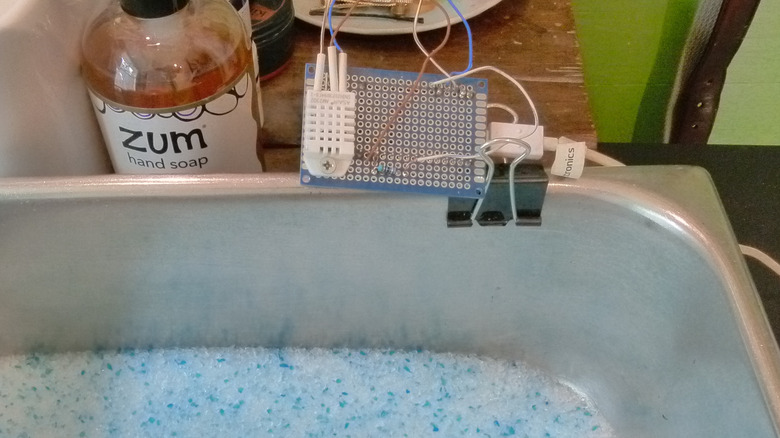
I weighed each pan of litter and placed it in a small, closed bathroom with the exhaust fan turned off. I ran a hot shower for 15 minutes, noting the room's humidity before and after the shower, and at 30-minute intervals thereafter, until the room's humidity returned to the pre-shower level. I hoped to learn how much water (as measured by weighing the litter at the end of the experiment) each medium adsorbed. I discontinued records once the humidity returned to the initial humidity (or lower), or if the rate of change was leveling off. The measurements were taken with a DHT22 temperature and humidity sensor attached to a ESP8266-based microcontroller and programmed with a simple web server to report the results.
So, I just let the bathroom dehumidify naturally with the door closed and the exhaust fan off, which is surely the worst-case scenario when it comes to dehumidifying a bathroom. If the cat litter was actually useful, a comparison with this approach should show it.
Two bags of cat litter walk into a bathroom...
I measured out four cups of each type of litter in a hotel steam table pan (586.4 grams of clay and 352 grams of silica gel). At the end of the experiment, I weighed the litter again to see how much water each managed to soak up. (Fortunately, it was water. A tray of cat litter set out in a household with two cats might have managed to collect other liquids.) The clay litter adsorbed 8.1 grams, 1.38% of the original weight, while the silica gel adsorbed 57.9 grams, 16.45% of the original weight. But naturally dehumidifying the bathroom without a fan happened within 15 minutes — faster than I expected and actually faster than the cat litter, probably because of a factor I didn't control for, like temperature or ambient humidity outside of the bathroom.
My results weren't promising. The silica gel litter adsorbed 16.45% of its own weight (just under 58g) in water before slowing to a near standstill, while the clay litter soaked up 1.38% (just over 8g). According to research from desiccant manufacturers and various scientific studies, bentonite clay can be expected to adsorb a maximum of 19.1% of its weight, and that is only in the most ideal of circumstances. Silica gel, on the other hand, can soak up as much as 40% of its dry weight in water. So both performed far below their hypothetical potential.
Two ways not to dehumidify a bathroom
The silica gel obviously did better. A five-pound bag of silica gel litter could, hypothetically, adsorb 2 pounds (907.2g) of water. Some back-of-the-napkin calculations suggest that a room of that size with a starting temperature of 86 degrees Fahrenheit could hold 270.65g of water, which is well within the silica gel's technical capacity to soak up ... but it doesn't, at least not quickly enough. At 77 degrees Fahrenheit, silica gel reaches its capacity to adsorb water after 4-5 hours; clay hits its limit at 13 hours or so. Both are far too long in an environment that's constantly exchanging air with other rooms and with the outside, which usually has an open source of water (the toilet bowl), and which occasionally has its humidity refreshed with baths, showers, and hand-washing.
Cat litter clearly isn't an efficient or cost-effective way of removing moisture from a room's air. In general, desiccants are used to dehumidify air in a very small, tightly sealed space like retail packaging. For example, Sorbent Systems' Dri-Box product is designed to desiccate 3 cubic feet of space with a 45-gram silica gel or a 35-gram zeolite cartridge. Bathrooms are, of course, much larger than this, and typically exchange air with other rooms and with the outdoors, leaving the desiccant with the task of dehumidifying the entire world, or dry trying. Fortunately, there are lots of ways to dehumidify a room without a dehumidifier, and most are more promising than cat litter.